Microbiological Lab Testing
We offer a wide range of microbiological, chemical and packaging tests including qPCR testing.
Pharmaceutical Packing
Ready to Use (RTU) options for vials, bottles, stoppers and caps. Products rinsed, depyrogenated, packed and sterilized.
Sterilisation
We are the largest independent sterilization company in the UK and Ireland offering both steam and ETO options for both small and large volumes.
Validation
Our expert validations team will work with you to develop the most suitable process to demonstrate compliance with relevant ISO standards.
Sterile Packing
Cleanroom assembly and packing in ISO 5 or 7 cleanrooms. Integrated service including cleaning, assembly, packing, sterilization and testing all in-house.
Popular Products
Latest News
The Importance of Spa & Swimming Pool Water Testing
In the realm of spa and swimming pool maintenance, water testing emerges as a crucial practice en...
What are Strabismus Scissors
Strabismus scissors are specialized surgical instruments crucial in ophthalmic procedures aimed a...
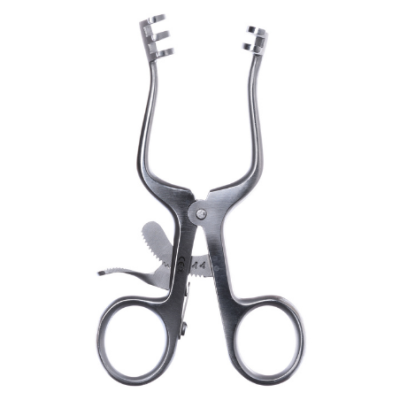
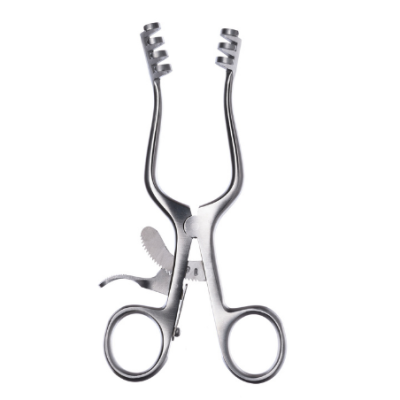
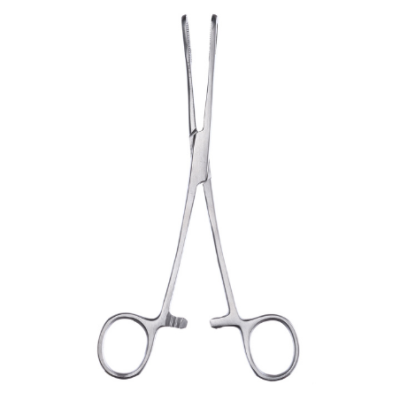
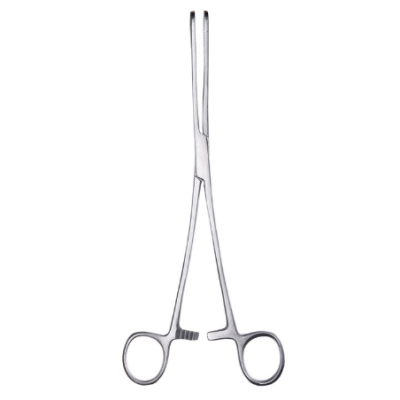
What are Spencer Wells Forceps
In the realm of surgery, Spencer Wells forceps stand as indispensable instruments, revered for th...
What are Tenotomy scissors
In the intricate realm of surgery, tenotomy scissors play a crucial role, renowned for their prec...
The Importance of Spa & Swimming Pool Water Testing
In the realm of spa and swimming pool maintenance, water testing emerges as a crucial practice en...
What are Strabismus Scissors
Strabismus scissors are specialized surgical instruments crucial in ophthalmic procedures aimed a...
What are Spencer Wells Forceps
In the realm of surgery, Spencer Wells forceps stand as indispensable instruments, revered for th...
What are Tenotomy scissors
In the intricate realm of surgery, tenotomy scissors play a crucial role, renowned for their prec...